OUR NEWS - RECENT NEWS
Roche Breast Cancer Study: Three Quebec Institutions in the Top 3 Worldwide
Roche Breast Cancer Study: Three Quebec Institutions in the Top 3 Worldwide Thanks to support from each of the FAST TRACK Evaluation Service’s stakeholders, three Quebec hospitals stood out on the international stage for the speed with which they implemented the second phase of Roche’s neoTOV breast cancer study. The Jewish General Hospital (JGH), with Dr. Stephanie Wong, was
CATALIS Celebrates Four Years of Success With the FAST TRACK Evaluation Service
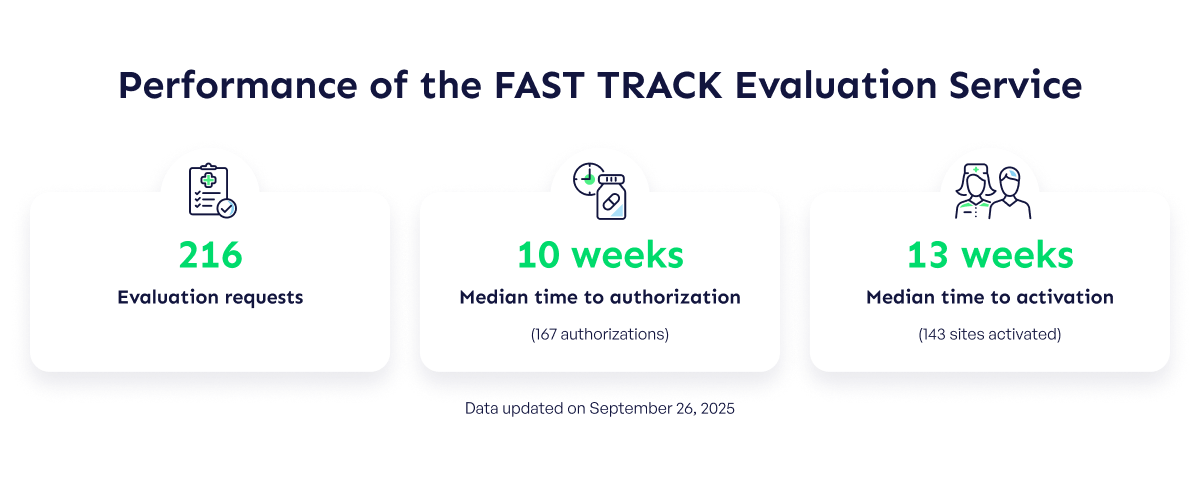
CATALIS Celebrates Four Years of Success With the FAST TRACK Evaluation Service CATALIS is proud to celebrate four years of success for the FAST TRACK Evaluation Service, which continues to demonstrate its effectiveness in optimizing administrative processes in clinical research in Quebec. Since the launch of the pilot project in 2021, 216 evaluation requests have been processed by the
Montreal’s Jewish General Hospital Authorizes a Novartis Study on GEP-NETs in Only 8 Weeks and Becomes the Second Site Activated Worldwide
Montreal’s Jewish General Hospital Authorizes a Novartis Study on GEP-NETs in Only 8 Weeks and Becomes the Second Site Activated Worldwide The Jewish General Hospital (JGH) in Montreal authorized Novartis’ NETTER-3 study in only 8 weeks with support from the FAST TRACK Evaluation Service. It was fully activated in only 10.2 weeks and allowed the team led by Dr.
CATALIS Unveils Its Clinical Trials Quebec Training Program
CATALIS Unveils Its Clinical Trials Quebec Training Program As part of our 2025–2026 action plan, CATALIS was tasked with promoting best practices in clinical research in Quebec by implementing a provincial training program for research professionals. On one hand, following recommendations from our Network, we renewed our partnership with N2 Network of Networks to provide Québec healthcare institutions with
CATALIS Honors Louise Proulx at the Conclusion of Her Term on the Board of Directors
CATALIS Honors Louise Proulx at the Conclusion of Her Term on the Board of Directors CATALIS wishes to express its gratitude and acknowledge the end of Ms. Louise Proulx’s term as a member of the Board of Directors and Chair of the Audit and Risk Management Committee. An active member of the Board since 2018, Ms. Proulx has played a key role in
Bayer Liver Cancer Study: 3 Quebec Institutions Rank Among the Top 3 Worldwide
Bayer Liver Cancer Study: 3 Quebec Institutions Rank Among the Top 3 Worldwide With support from the FAST TRACK Evaluation Service, three Quebec hospital research centers were positioned at the very top globally for the speed of implementation of a Phase I clinical study conducted by Bayer on hepatocellular carcinoma. The Centre hospitalier de l’Université de Montréal (CHUM), with
The MUHC Authorizes an AstraZeneca Study on Metastatic Lung Cancer in 8 Weeks and Recruits the First Patient in the World
The MUHC Authorizes an AstraZeneca Study on Metastatic Lung Cancer in 8 Weeks and Recruits the First Patient in the World With the support of the FAST TRACK Evaluation Service coordinated by CATALIS Quebec, the McGill University Health Centre (MUHC) was able to obtain authorization in 8 weeks for the ARTEMIDE-Lung03 study conducted by AstraZeneca, and was able to
Amgen Canada Obtains Authorization in 8 Weeks for Its First FAST TRACK Study on Lung Cancer at the McGill University Health Centre, Making It the First Site Activated in Canada in 9.8 weeks
Amgen Canada Obtains Authorization in 8 Weeks for Its First FAST TRACK Study on Lung Cancer at the McGill University Health Centre, Making It the First Site Activated in Canada in 9.8 weeks Amgen Canada used CATALIS Quebec’s FAST TRACK Evaluation Service for its 20230153 Phase II study on non-small cell lung cancer (NSCLC). Through this initiative, the research
Two Clinical Trials on Asthma Conducted by Sanofi Are Authorized in a Record Median Time of 6.7 Weeks at the CHUS, With the Support of the Fast Track Evaluation Service
Two Clinical Trials on Asthma Conducted by Sanofi Are Authorized in a Record Median Time of 6.7 Weeks at the CHUS, With the Support of the Fast Track Evaluation Service Sanofi used CATALIS’ FAST TRACK Evaluation Service for two Phase II studies, AIRCULES and AIRLYMPUS, focusing on asthma. The research team led by Dr. Simon Couillard of the Centre for
6 New Members Join the CATALIS Network
6 New Members Join the CATALIS Network CATALIS is proud to welcome four new HSSIs, one new pharmaceutical company, and one new patient organization that have just joined its extensive Network of partners. 4 New HSSIs Join the CATALIS Network CATALIS Quebec is proud to announce that the reach of its services now extends to 27 institutions
3 New Members on the CATALIS’ Board of Directors
3 New Members on the CATALIS’ Board of Directors CATALIS is pleased to announce the appointments of Alain Couture, Natalie Verreault and Patrick Montpetit to the Board of Directors as board members. The Board of Directors Welcomes Alain Couture “It is with great pleasure that I join the CATALIS Board of Directors. I am eager to
Update on Bill 5: New Tools Available for Health Institutions
Update on Bill 5: New Tools Available for Health Institutions As part of its government mandate to optimize the clinical research environment in Quebec, CATALIS supports provincial efforts that focus on the implementation of the Bill 5 on health and social services information (AHSSI or Bill 5) . Its objective is to facilitate effective access to health information for
Five New Health Institutions Join the FAST TRACK Evaluation Service
Five New Health Institutions Join the FAST TRACK Evaluation Service The CATALIS Network continues to grow and collaborate to accelerate clinical research in Quebec. We proudly announce that five new health and social services institutions (HSSIs) will soon offer the FAST TRACK Evaluation Service. The CISSS de Lanaudière, the CISSS des Laurentides, the CISSS de Laval, the CISSS de
The CHUM Authorizes a Novartis Study on Prostate Cancer With the Support of the FAST TRACK Evaluation Service and Recruits the First Patient Worldwide
The CHUM Authorizes a Novartis Study on Prostate Cancer With the Support of the FAST TRACK Evaluation Service and Recruits the First Patient Worldwide Novartis used the FAST TRACK Evaluation Service for its phase III study, PSMA-DC, which aims to assess the efficacy and safety of lutetium vipivotide tetraxetan (AAA617) in patients whose prostate cancer has advanced to an
The CHU de Québec-Université Laval Recruits the First Two Participants Worldwide for a Clinical Trial on Breast Cancer by Roche, With the Support of the FAST TRACK Evaluation Service
The CHU de Québec-Université Laval Recruits the First Two Participants Worldwide for a Clinical Trial on Breast Cancer by Roche, With the Support of the FAST TRACK Evaluation Service The research team led by Dr. Julie Lemieux of CHU de Québec-Université Laval recently recruited the first two participants worldwide for a phase III breast cancer study. With the support
Montreal’s Jewish General Hospital Authorized the First BMS Study in 8.6 Weeks and Is the Most Rapidly Activated Site in Canada
Montreal’s Jewish General Hospital Authorized the First BMS Study in 8.6 Weeks and Is the Most Rapidly Activated Site in Canada The Bristol Mayers Squibb (BMS) pharmaceutical company used the FAST TRACK Evaluation Service for the first time, which led to the Jewish General Hospital’s Dr. Sarit Assouline’s research team to obtain authorization for the CA235-0001 study in a
The Amgen Pharmaceutical Company Joins the CATALIS Network
The Amgen Pharmaceutical Company Joins the CATALIS Network CATALIS is pleased to welcome the Amgen pharmaceutical company to its Network of Partners. This biotechnology industry pioneer is dedicated to developing new treatments for various conditions, including rare diseases. Amgen joins the many industry partners who are involved in the Network: Abbvie, Alexion, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Bristol Myers
3 New Patient Organizations Join the CATALIS Network
3 New Patient Organizations Join the CATALIS Network We are pleased to announce that three new organizations have joined the CATALIS Network: The Canadian Association of Paroxysmal Nocturnal Haemoglobinuria (PNH) Patients, Crohn’s and Colitis Canada, and Pulmonary Hypertension Association Canada (PHA). These new members join the more than thirty patient-partner organizations currently involved in our Network. The CATALIS Network
Renewed Partnership: CATALIS Quebec Partners With N2 Canada to Offer a Membership for 2024-2025 to Health and Social Services Network Institutions
Renewed Partnership: CATALIS Quebec Partners With N2 Canada to Offer a Membership for 2024-2025 to Health and Social Services Network Institutions CATALIS Quebec is proud to announce the renewal of its partnership with N2 Canada for 2024-2025. Through this partnership, CATALIS Quebec has committed to supporting the 2024-2025 membership of the health and social services institutions (HSSIs) which are
The CATALIS Network Rolls Out a New Strategic Plan for 2024-2028
The CATALIS Network Rolls Out a New Strategic Plan for 2024-2028 The CATALIS Quebec Network is pleased to announce the rollout of its new 2024-2028 Strategic Plan in collaboration with its partners, the Ministry of Health and Social Services (MHSS) and the Ministry of Economy, Innovation and Energy (MEIE). An Innovative and Ambitious Strategic Plan for 2024-2028
The CATALIS Network Supports Provincial Efforts for an Even More Innovative and Productive Clinical Research Environment
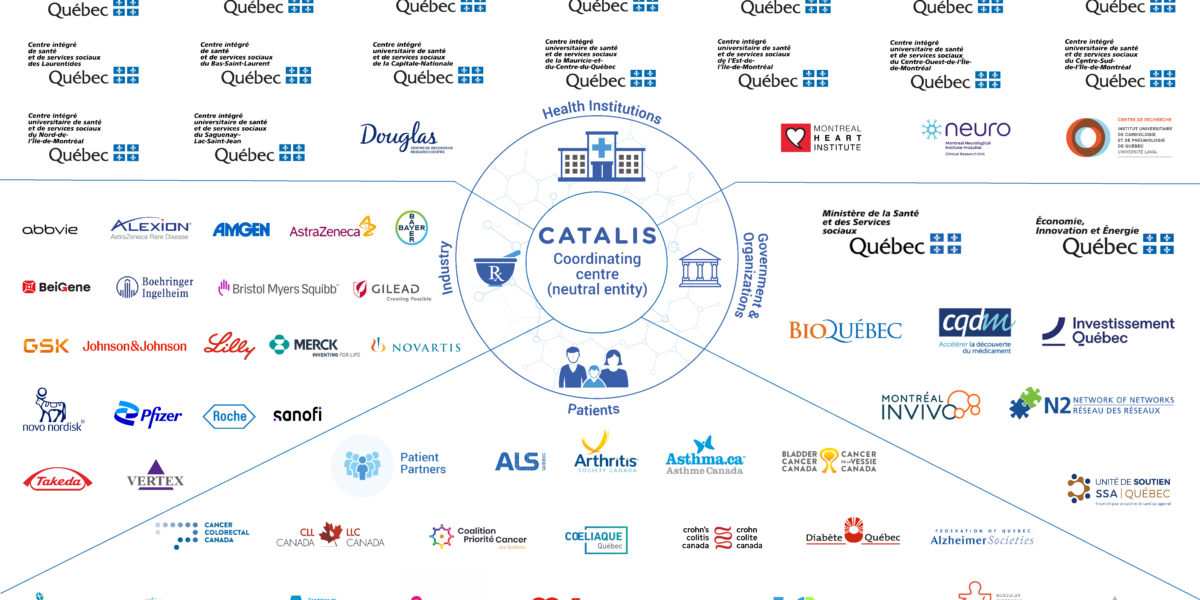
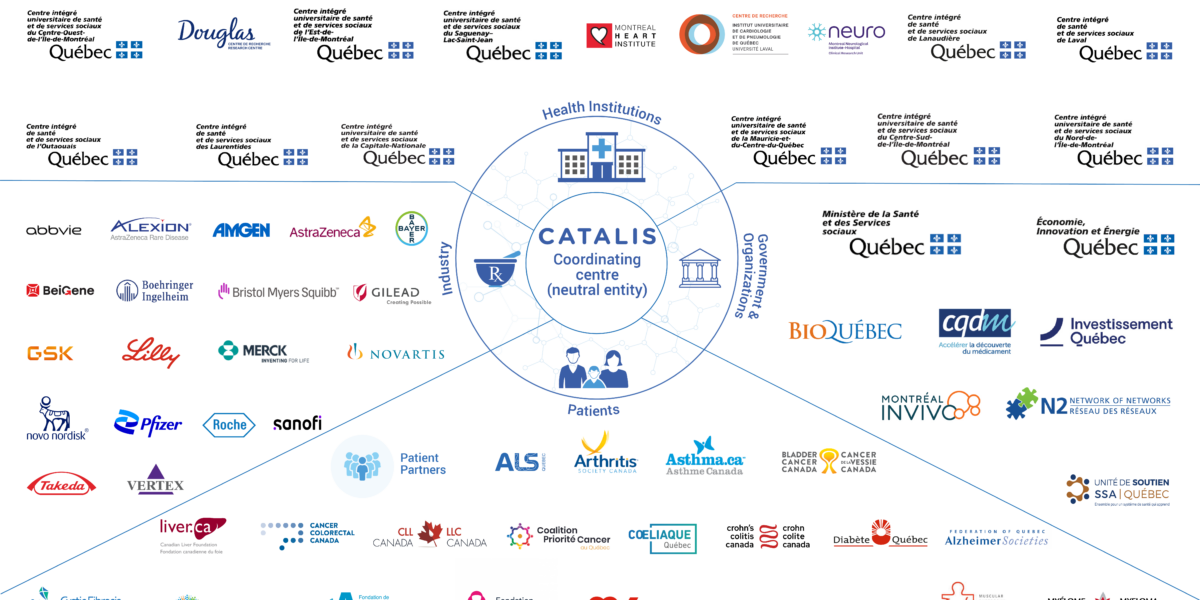
The CATALIS Network Supports Provincial Efforts for an Even More Innovative and Productive Clinical Research Environment Since its inception, CATALIS Quebec’s government mandate has been to act as a neutral and agile entity for mobilizing and coordinating efforts aimed at optimizing Quebec’s clinical research environment. CATALIS has facilitated more than 280 advisory committees and working groups with members of
3 Pharmaceutical Companies Join the CATALIS Network
3 Pharmaceutical Companies Join the CATALIS Network CATALIS is pleased to welcome the pharmaceutical companies Alexion, Lilly Canada and Novo Nordisk Canada Inc. to its Network of partners. These companies join the many industry partners committed to the Network, namely Abbvie, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Gilead Sciences Canada, GSK, Merck, Novartis, Pfizer, Roche, Sanofi, Takeda,
2 Patient Organizations Join the CATALIS Network
2 Patient Organizations Join the CATALIS Network New organizations join the CATALIS Network: Asthma Canada and Kidney Cancer Canada. They join more than 30 patient organizations and patient partners who represent the patient’s voice within the Network and contribute to CATALIS’ mission of optimizing clinical research in Quebec, in order to accelerate the development of innovative patient care for
The Chaudière-Appalaches Integrated Health and Social Services Centre Authorized a First Clinical Trial in 8.4 Weeks With the FAST TRACK Evaluation Service and Recruited the First Canadian Participant
The Chaudière-Appalaches Integrated Health and Social Services Centre Authorized a First Clinical Trial in 8.4 Weeks With the FAST TRACK Evaluation Service and Recruited the First Canadian Participant The very first collaboration between the Chaudière-Appalaches Centre intégré de santé et de services sociaux (integrated health and social services centre or CISSS) and CATALIS’ FAST TRACK Evaluation Service has been