OUR NEWS - FAST TRACK SERVICE
Roche Breast Cancer Study: Three Quebec Institutions in the Top 3 Worldwide
Roche Breast Cancer Study: Three Quebec Institutions in the Top 3 Worldwide Thanks to support from each of the FAST TRACK Evaluation Service’s stakeholders, three Quebec hospitals stood out on the international stage for the speed with which they implemented the second phase of Roche’s neoTOV breast cancer study. The Jewish General Hospital (JGH), with Dr. Stephanie Wong, was
CATALIS Celebrates Four Years of Success With the FAST TRACK Evaluation Service
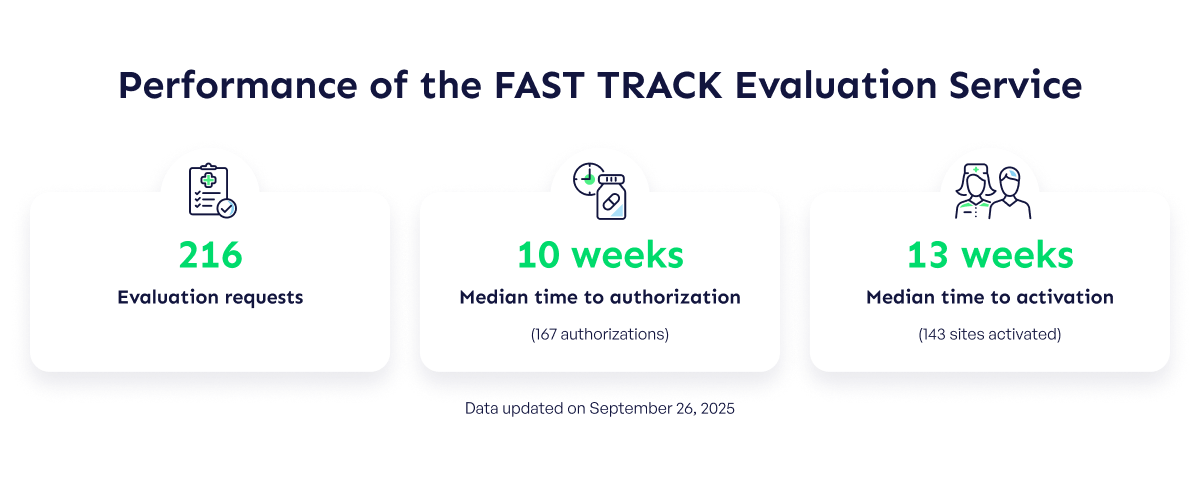
CATALIS Celebrates Four Years of Success With the FAST TRACK Evaluation Service CATALIS is proud to celebrate four years of success for the FAST TRACK Evaluation Service, which continues to demonstrate its effectiveness in optimizing administrative processes in clinical research in Quebec. Since the launch of the pilot project in 2021, 216 evaluation requests have been processed by the
Montreal’s Jewish General Hospital Authorizes a Novartis Study on GEP-NETs in Only 8 Weeks and Becomes the Second Site Activated Worldwide
Montreal’s Jewish General Hospital Authorizes a Novartis Study on GEP-NETs in Only 8 Weeks and Becomes the Second Site Activated Worldwide The Jewish General Hospital (JGH) in Montreal authorized Novartis’ NETTER-3 study in only 8 weeks with support from the FAST TRACK Evaluation Service. It was fully activated in only 10.2 weeks and allowed the team led by Dr.
Bayer Liver Cancer Study: 3 Quebec Institutions Rank Among the Top 3 Worldwide
Bayer Liver Cancer Study: 3 Quebec Institutions Rank Among the Top 3 Worldwide With support from the FAST TRACK Evaluation Service, three Quebec hospital research centers were positioned at the very top globally for the speed of implementation of a Phase I clinical study conducted by Bayer on hepatocellular carcinoma. The Centre hospitalier de l’Université de Montréal (CHUM), with
Five New Health Institutions Join the FAST TRACK Evaluation Service
Five New Health Institutions Join the FAST TRACK Evaluation Service The CATALIS Network continues to grow and collaborate to accelerate clinical research in Quebec. We proudly announce that five new health and social services institutions (HSSIs) will soon offer the FAST TRACK Evaluation Service. The CISSS de Lanaudière, the CISSS des Laurentides, the CISSS de Laval, the CISSS de
The CHUM Authorizes a Novartis Study on Prostate Cancer With the Support of the FAST TRACK Evaluation Service and Recruits the First Patient Worldwide
The CHUM Authorizes a Novartis Study on Prostate Cancer With the Support of the FAST TRACK Evaluation Service and Recruits the First Patient Worldwide Novartis used the FAST TRACK Evaluation Service for its phase III study, PSMA-DC, which aims to assess the efficacy and safety of lutetium vipivotide tetraxetan (AAA617) in patients whose prostate cancer has advanced to an
The CHU de Québec-Université Laval Recruits the First Two Participants Worldwide for a Clinical Trial on Breast Cancer by Roche, With the Support of the FAST TRACK Evaluation Service
The CHU de Québec-Université Laval Recruits the First Two Participants Worldwide for a Clinical Trial on Breast Cancer by Roche, With the Support of the FAST TRACK Evaluation Service The research team led by Dr. Julie Lemieux of CHU de Québec-Université Laval recently recruited the first two participants worldwide for a phase III breast cancer study. With the support
Montreal’s Jewish General Hospital Authorized the First BMS Study in 8.6 Weeks and Is the Most Rapidly Activated Site in Canada
Montreal’s Jewish General Hospital Authorized the First BMS Study in 8.6 Weeks and Is the Most Rapidly Activated Site in Canada The Bristol Mayers Squibb (BMS) pharmaceutical company used the FAST TRACK Evaluation Service for the first time, which led to the Jewish General Hospital’s Dr. Sarit Assouline’s research team to obtain authorization for the CA235-0001 study in a
The Chaudière-Appalaches Integrated Health and Social Services Centre Authorized a First Clinical Trial in 8.4 Weeks With the FAST TRACK Evaluation Service and Recruited the First Canadian Participant
The Chaudière-Appalaches Integrated Health and Social Services Centre Authorized a First Clinical Trial in 8.4 Weeks With the FAST TRACK Evaluation Service and Recruited the First Canadian Participant The very first collaboration between the Chaudière-Appalaches Centre intégré de santé et de services sociaux (integrated health and social services centre or CISSS) and CATALIS’ FAST TRACK Evaluation Service has been
Bayer: The CHUM Was Ranked First in the World After Using the FAST TRACK Evaluation Service for the First Time
Bayer: The CHUM Was Ranked First in the World After Using the FAST TRACK Evaluation Service for the First Time The Bayer pharmaceutical company used the FAST TRACK Evaluation Service for the first time to accelerate the authorization of its PAnTHA advanced prostate cancer trial at the Centre hospitalier de l’Université de Montréal (CHUM) in 10.4 weeks, with site
A New Brand Identity for the FAST TRACK Evaluation Service
Almost 2 years after its inauguration, the FAST TRACK Evaluation Service is unveiling a new brand identity. Officially launched by the CATALIS Network in September 2022, the FAST TRACK Evaluation Service aims to authorize clinical trials in ≤ 8 weeks. We are pleased to announce that, to date, the service has enabled the authorization of 37 clinical trials conducted by
BeiGene: A First Trial Authorized With a Median Time of 8.1 Weeks
BeiGene: A First Trial Authorized With a Median Time of 8.1 Weeks Thanks to CATALIS’ FAST TRACK Evaluation Service, the BeiGene pharmaceutical company accelerated the authorization for its trial on chronic lymphoid leukemia (BGB-11417-301) in 3 Quebec hospitals with a median time of 8.1 weeks. In fact, the CHU-Sherbrooke authorized the trial in 7.8 weeks and became the first site
GSK Authorized 3 First Trials With a Median Time of 8.4 Weeks and Activated the First Site Globally
GSK Authorized 3 First Trials With a Median Time of 8.4 Weeks and Activated the First Site Globally Thanks to CATALIS’ FAST TRACK Evaluation Service and its participating healthcare institutions, the GlaxoSmithKline Inc. (GSK) pharmaceutical company was able to accelerate the authorization of three clinical trials, with a record median time of 8.4 weeks. The Galaxies 202 trial, which
First Pfizer Pharmaceutical Company Trial Authorized With a Median of 8.2 Weeks Using the FAST TRACK Evaluation Service
First Pfizer Pharmaceutical Company Trial Authorized With a Median of 8.2 Weeks Using the FAST TRACK Evaluation Service For the first time, the Pfizer pharmaceutical company used CATALIS’ FAST TRACK Evaluation Service to authorize a complex Phase Ia/Ib oncology clinical trial, meaning a master protocol that includes several sub-studies. In this study, Pfizer is assessing the effectiveness of a
Catalis in Support of the Quebec Government’s Policy on Rare Diseases
CATALIS in Support of the Quebec Government’s Policy on Rare Diseases On June 6, the Quebec government unveiled its Politique québécoise pour les maladies rares (Quebec policy on rare diseases – in French only). In Quebec, 700,000 people with rare diseases, half of whom are children, face significant challenges such as errors in diagnosing their disease and the absence
MASter-1 Study: Authorization in 8 Weeks to Explore a New Treatment for an Extremely Rare Disease
MASter-1 Study: Authorization in 8 Weeks to Explore a New Treatment for an Extremely Rare Disease As mentioned in our December 2022 newsletter, CATALIS is proud to support the Quebec government’s Politique québécoise sur les maladies rares (Quebec policy on rare diseases – in French only), namely by allowing studies to be activated more quickly thanks to its FAST
STRENGTH Study: Zolgensma for Children Aged 2 to 12 With Spinal Muscular Atrophy
STRENGTH Study: Zolgensma for Children Aged 2 to 12 With Spinal Muscular Atrophy In October 2021, Minister Dubé announced that Zolgensma, a drug produced by the Novartis pharmaceutical company, was added to the list of drugs offered in Quebec health facilities to treat children under the age of 2 with spinal muscular atrophy. Spinal muscular atrophy is a rare
NEPTUNUS-2 Study: Sjogren’s Syndrome Clinical Trial Approved in 8 Weeks via CATALIS’ FAST TRACK Evaluation Service
NEPTUNUS-2 Study: Sjogren’s Syndrome Clinical Trial Approved in 8 Weeks via CATALIS’ FAST TRACK Evaluation Service As mentioned in our December 2022 newsletter, CATALIS is proud to announce that its FAST TRACK Evaluation Service continues to allow industry-financed studies to be authorized in record time. In NEPTUNUS-2, a phase III study, Novartis and Dr. Isabelle Fortin are testing the
ONWARDS Study: Authorization in 9 Weeks for Symptomatic Osteoarthritis of the Knee Study via CATALIS’ FAST TRACK Evaluation Service
ONWARDS Study: Authorization in 9 Weeks for Symptomatic Osteoarthritis of the Knee Study via CATALIS’ FAST TRACK Evaluation Service In addition to the 3 rare disease studies, a 4th Novartis study on symptomatic osteoarthritis of the knee was authorized by the Centre hospitalier universitaire de Sherbrooke (CIUSSS de l’Estrie – CHUS) in record time via the CATALIS FAST TRACK
Sanofi Pharmaceutical Company’s First Clinical Trial Approved Through CATALIS Quebec’s FAST TRACK Evaluation Service
Sanofi Pharmaceutical Company’s First Clinical Trial Approved Through CATALIS Quebec’s FAST TRACK Evaluation Service Following Novartis, Boehringer, and Roche, Sanofi is now CATALIS’ fourth pharmaceutical partner to pilot its FAST TRACK Evaluation Service. Sanofi’s Phase I-ll study, which uses human subjects for the first time and is designed to assess the safety and the pharmacokinetic, pharmacodynamic, and antitumor activity
Roche Pharmaceutical Company’s First Clinical Trial Approved Through CATALIS Quebec’s FAST TRACK Evaluation Service
Roche Pharmaceutical Company’s First Clinical Trial Approved Through CATALIS Quebec’s FAST TRACK Evaluation Service Thanks to CATALIS’ FAST TRACK evaluation service, Roche’s ProHer multi-centre study, which explores a new treatment method for patients with breast cancer, was approved in 10 weeks by the CHU de Québec-Université Laval. More specifically, this study seeks to discover patients’ preference for at-home administration
Two New Projects Successfully Authorized Within Seven Weeks Thanks to CATALIS QUEBEC’S FAST TRACK Evaluation Service
Two New Projects Successfully Authorized Within Seven Weeks Thanks to CATALIS QUEBEC’S FAST TRACK Evaluation Service While the first-ever FAST TRACK evaluation service pilot project was successfully completed last December, CATALIS is proud to announce that two more clinical trials have now been approved in record time. The second FAST TRACK evaluation service coordinated a Phase I oncology study
A First Successful Pilot Project for CATALIS’ New FAST TRACK Evaluation Service: 4 Quebec Institutions Ranked in the Top 5 of the World’s Fastest Sites
A First Successful Pilot Project for CATALIS’ New FAST TRACK Evaluation Service: 4 Quebec Institutions Ranked in the Top 5 of the World’s Fastest Sites CATALIS is very proud to announce that the first pilot of its new FAST TRACK assessment service has successfully reduced the approval time for an industry-financed clinical trial by almost 75%. In September 2021,
CATALIS Is Piloting Its New FAST TRACK Evaluation Service
The CATALIS Public-Private Network has developed a new FAST TRACK evaluation service that will eventually allow industry-funded clinical trials to be authorized in less than 8 weeks.