News
3 New Members on the CATALIS’ Board of Directors
3 New Members on the CATALIS’ Board of Directors CATALIS is pleased to announce the appointments of Alain Couture, Natalie Verreault and Patrick Montpetit to the Board of Directors as board members. The Board of Directors Welcomes Alain Couture “It is with great pleasure that I join the CATALIS Board of Directors. I am eager to
Update on Bill 5: New Tools Available for Health Institutions
Update on Bill 5: New Tools Available for Health Institutions As part of its government mandate to optimize the clinical research environment in Quebec, CATALIS supports provincial efforts that focus on the implementation of the Bill 5 on health and social services information (AHSSI or Bill 5) . Its objective is to facilitate effective access to health information for
Five New Health Institutions Join the FAST TRACK Evaluation Service
Five New Health Institutions Join the FAST TRACK Evaluation Service The CATALIS Network continues to grow and collaborate to accelerate clinical research in Quebec. We proudly announce that five new health and social services institutions (HSSIs) will soon offer the FAST TRACK Evaluation Service. The CISSS de Lanaudière, the CISSS des Laurentides, the CISSS de Laval, the CISSS de
The CHUM Authorizes a Novartis Study on Prostate Cancer With the Support of the FAST TRACK Evaluation Service and Recruits the First Patient Worldwide
The CHUM Authorizes a Novartis Study on Prostate Cancer With the Support of the FAST TRACK Evaluation Service and Recruits the First Patient Worldwide Novartis used the FAST TRACK Evaluation Service for its phase III study, PSMA-DC, which aims to assess the efficacy and safety of lutetium vipivotide tetraxetan (AAA617) in patients whose prostate cancer has advanced to an
The CHU de Québec-Université Laval Recruits the First Two Participants Worldwide for a Clinical Trial on Breast Cancer by Roche, With the Support of the FAST TRACK Evaluation Service
The CHU de Québec-Université Laval Recruits the First Two Participants Worldwide for a Clinical Trial on Breast Cancer by Roche, With the Support of the FAST TRACK Evaluation Service The research team led by Dr. Julie Lemieux of CHU de Québec-Université Laval recently recruited the first two participants worldwide for a phase III breast cancer study. With the support
Montreal’s Jewish General Hospital Authorized the First BMS Study in 8.6 Weeks and Is the Most Rapidly Activated Site in Canada
Montreal’s Jewish General Hospital Authorized the First BMS Study in 8.6 Weeks and Is the Most Rapidly Activated Site in Canada The Bristol Mayers Squibb (BMS) pharmaceutical company used the FAST TRACK Evaluation Service for the first time, which led to the Jewish General Hospital’s Dr. Sarit Assouline’s research team to obtain authorization for the CA235-0001 study in a
The Amgen Pharmaceutical Company Joins the CATALIS Network
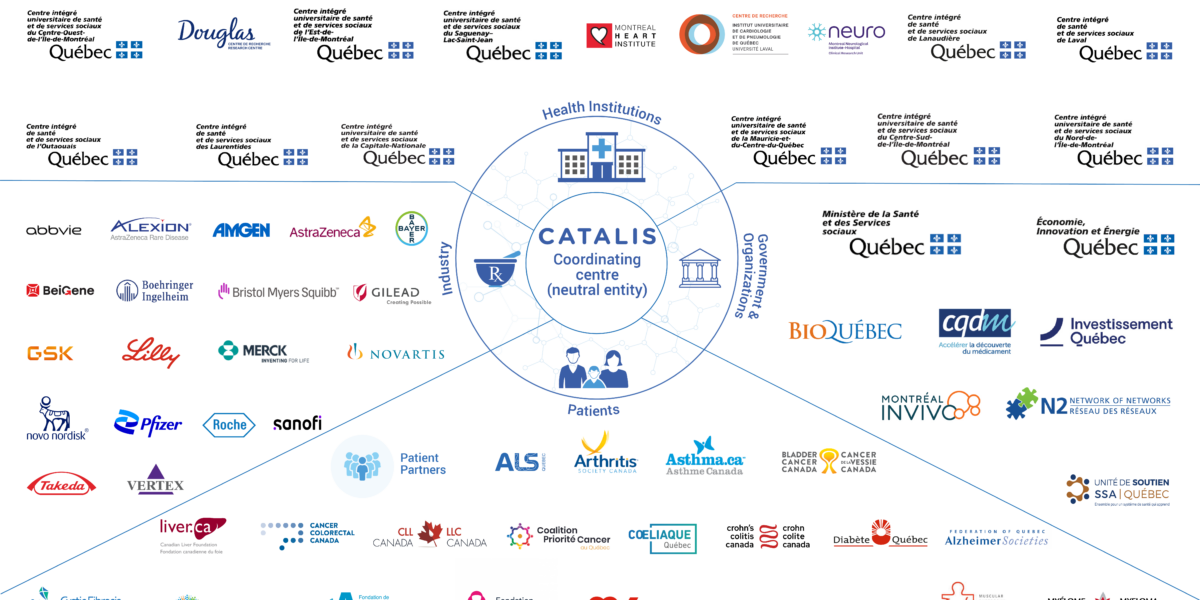
The Amgen Pharmaceutical Company Joins the CATALIS Network CATALIS is pleased to welcome the Amgen pharmaceutical company to its Network of Partners. This biotechnology industry pioneer is dedicated to developing new treatments for various conditions, including rare diseases. Amgen joins the many industry partners who are involved in the Network: Abbvie, Alexion, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Bristol Myers
3 New Patient Organizations Join the CATALIS Network
3 New Patient Organizations Join the CATALIS Network We are pleased to announce that three new organizations have joined the CATALIS Network: The Canadian Association of Paroxysmal Nocturnal Haemoglobinuria (PNH) Patients, Crohn’s and Colitis Canada, and Pulmonary Hypertension Association Canada (PHA). These new members join the more than thirty patient-partner organizations currently involved in our Network. The CATALIS Network
Renewed Partnership: CATALIS Quebec Partners With N2 Canada to Offer a Membership for 2024-2025 to Health and Social Services Network Institutions
Renewed Partnership: CATALIS Quebec Partners With N2 Canada to Offer a Membership for 2024-2025 to Health and Social Services Network Institutions CATALIS Quebec is proud to announce the renewal of its partnership with N2 Canada for 2024-2025. Through this partnership, CATALIS Quebec has committed to supporting the 2024-2025 membership of the health and social services institutions (HSSIs) which are
The CATALIS Network Rolls Out a New Strategic Plan for 2024-2028
The CATALIS Network Rolls Out a New Strategic Plan for 2024-2028 The CATALIS Quebec Network is pleased to announce the rollout of its new 2024-2028 Strategic Plan in collaboration with its partners, the Ministry of Health and Social Services (MHSS) and the Ministry of Economy, Innovation and Energy (MEIE). An Innovative and Ambitious Strategic Plan for 2024-2028