News
Roche Breast Cancer Study: Three Quebec Institutions in the Top 3 Worldwide
Roche Breast Cancer Study: Three Quebec Institutions in the Top 3 Worldwide Thanks to support from each of the FAST TRACK Evaluation Service’s stakeholders, three Quebec hospitals stood out on the international stage for the speed with which they implemented the second phase of Roche’s neoTOV breast cancer study. The Jewish General Hospital (JGH), with Dr. Stephanie Wong, was
CATALIS Celebrates Four Years of Success With the FAST TRACK Evaluation Service
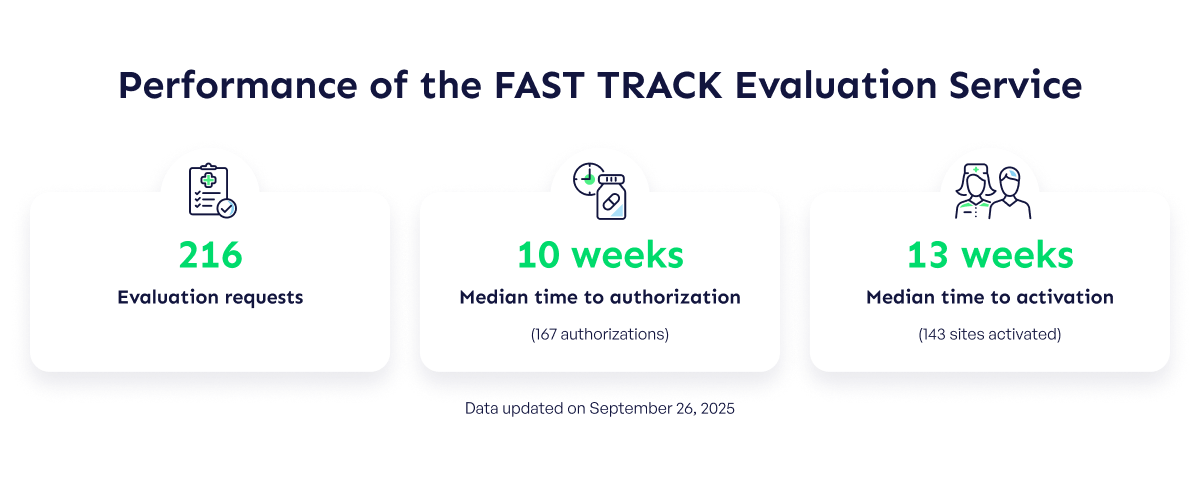
CATALIS Celebrates Four Years of Success With the FAST TRACK Evaluation Service CATALIS is proud to celebrate four years of success for the FAST TRACK Evaluation Service, which continues to demonstrate its effectiveness in optimizing administrative processes in clinical research in Quebec. Since the launch of the pilot project in 2021, 216 evaluation requests have been processed by the
Montreal’s Jewish General Hospital Authorizes a Novartis Study on GEP-NETs in Only 8 Weeks and Becomes the Second Site Activated Worldwide
Montreal’s Jewish General Hospital Authorizes a Novartis Study on GEP-NETs in Only 8 Weeks and Becomes the Second Site Activated Worldwide The Jewish General Hospital (JGH) in Montreal authorized Novartis’ NETTER-3 study in only 8 weeks with support from the FAST TRACK Evaluation Service. It was fully activated in only 10.2 weeks and allowed the team led by Dr.
CATALIS Unveils Its Clinical Trials Quebec Training Program
CATALIS Unveils Its Clinical Trials Quebec Training Program As part of our 2025–2026 action plan, CATALIS was tasked with promoting best practices in clinical research in Quebec by implementing a provincial training program for research professionals. On one hand, following recommendations from our Network, we renewed our partnership with N2 Network of Networks to provide Québec healthcare institutions with
CATALIS Honors Louise Proulx at the Conclusion of Her Term on the Board of Directors
CATALIS Honors Louise Proulx at the Conclusion of Her Term on the Board of Directors CATALIS wishes to express its gratitude and acknowledge the end of Ms. Louise Proulx’s term as a member of the Board of Directors and Chair of the Audit and Risk Management Committee. An active member of the Board since 2018, Ms. Proulx has played a key role in
Bayer Liver Cancer Study: 3 Quebec Institutions Rank Among the Top 3 Worldwide
Bayer Liver Cancer Study: 3 Quebec Institutions Rank Among the Top 3 Worldwide With support from the FAST TRACK Evaluation Service, three Quebec hospital research centers were positioned at the very top globally for the speed of implementation of a Phase I clinical study conducted by Bayer on hepatocellular carcinoma. The Centre hospitalier de l’Université de Montréal (CHUM), with
The MUHC Authorizes an AstraZeneca Study on Metastatic Lung Cancer in 8 Weeks and Recruits the First Patient in the World
The MUHC Authorizes an AstraZeneca Study on Metastatic Lung Cancer in 8 Weeks and Recruits the First Patient in the World With the support of the FAST TRACK Evaluation Service coordinated by CATALIS Quebec, the McGill University Health Centre (MUHC) was able to obtain authorization in 8 weeks for the ARTEMIDE-Lung03 study conducted by AstraZeneca, and was able to
Amgen Canada Obtains Authorization in 8 Weeks for Its First FAST TRACK Study on Lung Cancer at the McGill University Health Centre, Making It the First Site Activated in Canada in 9.8 weeks
Amgen Canada Obtains Authorization in 8 Weeks for Its First FAST TRACK Study on Lung Cancer at the McGill University Health Centre, Making It the First Site Activated in Canada in 9.8 weeks Amgen Canada used CATALIS Quebec’s FAST TRACK Evaluation Service for its 20230153 Phase II study on non-small cell lung cancer (NSCLC). Through this initiative, the research
Two Clinical Trials on Asthma Conducted by Sanofi Are Authorized in a Record Median Time of 6.7 Weeks at the CHUS, With the Support of the Fast Track Evaluation Service
Two Clinical Trials on Asthma Conducted by Sanofi Are Authorized in a Record Median Time of 6.7 Weeks at the CHUS, With the Support of the Fast Track Evaluation Service Sanofi used CATALIS’ FAST TRACK Evaluation Service for two Phase II studies, AIRCULES and AIRLYMPUS, focusing on asthma. The research team led by Dr. Simon Couillard of the Centre for
6 New Members Join the CATALIS Network
6 New Members Join the CATALIS Network CATALIS is proud to welcome four new HSSIs, one new pharmaceutical company, and one new patient organization that have just joined its extensive Network of partners. 4 New HSSIs Join the CATALIS Network CATALIS Quebec is proud to announce that the reach of its services now extends to 27 institutions